ペプチドミクス研究部 Department of Peptidomics
メンバー
水平方向にスクロールできます。
| 部長(PI) | |||
| 研究員 | 中村 真男 | Masao Nakamura | Researchmap メールアドレス m-nakamura |
| 研究助手 | |||
| 客員研究員 | 佐々木 一樹 | Kazuki Sasaki | Researchmap メールアドレス k-sasaki |
※メールアドレスには@po.kyoundo.jpをつけて下さい
研究内容
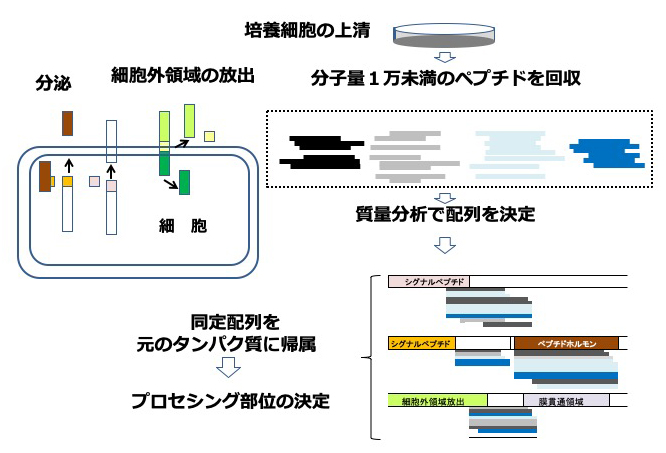
ペプチドミクスは、細胞や組織に見出される分子量1万未満のペプチドを包括的に調べる研究分野です。この領域は電気泳動で調べにくい大きさで、質量分析法の発達にも関わらず、細胞間の調節因子として働くペプチドホルモンや抗菌ペプチド以外は、その実態がよくわかっていませんでした。当研究部では、この領域を調べることによって、分泌タンパク質や膜タンパク質のプロセシング部位を明らかにできることを提唱しています。タンパク質のプロセシングとは、特定の酵素が働いてタンパク質を切断することですが、分解的な切断とは異なる意味をもっています。プロセシングによって、生理活性を持つ分子がタンパク質の内側から生じたり、リガンドを捕捉する可溶型受容体が生じます。プロセシングの場所は、配列だけではなく、糖鎖などの修飾によっても影響を受けるので、in silicoでの予測はおのずと限界があり、実際の配列を調べることで初めて明らかになります。特定できたプロセシング部位は、診断や治療の新しいターゲットを見出すための有用な情報となります。実際に、これまでに新しいヒトの生理活性ペプチドを複数見出し、新規ペプチドの探索に質量分析を応用した世界初の事例となっています。浸潤性膵管癌で発現する分泌タンパク質と膜タンパク質の切断部位の特定を進めて、この難治がんの診断や治療に役立つ手がかりを見出す研究を進めています。
研究業績
過去5年間の発表論文
- Zhang W#, Miura A#, Abu Saleh MM, Shimizu K, Mita Y, Tanida R, Hirako S, Shioda S, Gmyr V, Kerr-Conte J, Pattou F, Jin C, Kanai Y, Sasaki K, Minamino N, Sakoda H, Nakazato M: The NERP-4-SNAT2 axis regulates pancreatic β-cell maintenance and function #Contributed equally. Nature Commun 14: 8158, 2023.
- Noguchi R, Kosako H, Sasaki K, Kondo T: Data for proteogenomic analysis of two patient-derived undifferentiated pleomorphic sarcoma cell lines. J Proteome Data and Methods 5: 13, 2023.
- Nakamura M, Shiga A, Iimori A, Matsuzaki T: Efficient endocytosis of the human lactoferrin N-lobe enhances its antiproliferative activity against human cancer cells. Biol Pharm Bull. 46: 1004-1009, 2023.
- Paudel D, Kuramitsu Y, Uehara O, Morikawa T, Yoshida K, Giri S, Islam S, Kitagawa T, Kondo T, Sasaki K, Matsuoka H, Miura H, Abiko Y: Proteomic and microbiota analyses of the oral cavity during psychological stress. PLoS ONE 17: e0268155, 2022.
- Nakamura M, Sato A: Glycan-binding properties of basic whey protein lactoferrin and its application in nerve regenerative medicine. Trends Glycosci. Glycotechnol. 34: E19-E23, 2022.
- Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, Yanagihara K, Imai T, Ohki R, Yashiro M, Tanaka M, Sakai R, Yamaguchi H: Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Lett. 526: 335-345, 2022.
- Nakamura M: Development of a highly functionalized lactoferrin that controls nerve function: Fusion of glycan-binding ability and neuroprotective function. Glycoforum 24: A8, 2021.
- Nakamura M, Matsuzaki T, Iimori A and Sato A: Harnessing the chondroitin sulfate-binding characteristics of human lactoferrin to neutralize neurite outgrowth inhibition. Biochem. Biophys. Res. Commun. 534: 1076-1082, 2021.
- Nagamura Y, Miyazaki M, Nagano Y, Yuki M, Fukami K, Yanagihara K, Sasaki K, Sakai R, and Yamaguchi H: PLEKHA5 regulates the survival and peritoneal dissemination of diffuse-type gastric carcinoma cells with Met gene. Oncogenesis 10: 25, 2021.
- Miyazaki S, Tashiro F, Tsuchiya T, Sasaki K and Miyazaki JI: Establishment of a long-term stable β-cell line and its application to analyze the effect of Gcg expression on insulin secretion. Sci. Rep. 11: 477, 2021.
- Tsuchiya T, Nakayama A, Kawamura T and Sasaki K: Capillary electrophoresis electrospray ionization-mass spectrometry for peptidomics-based processing site determination. Biochem. Biophys. Res. Commun. 533: 872-878, 2020.
- Ueda K, Shimizu M, Ohashi A, Murata D, Suzuki T, Kobayashi N, Baba J, Takeuchi T, Shiga Y, Nakamura M, Kagaya S and Sato A: Albumin fusion at the N-terminus or C-terminus of human lactoferrin leads to improved pharmacokinetics and anti-proliferative effects on cancer cell lines. Eur. J. Pharm. Sci. 155: 10551, 2020.
- Matsuzaki T, Nakamura M, Nogita T and Sato A: Cellular uptake and release of intact lactoferrin and its derivatives in an intestinal enterocyte model of Caco-2 cells. Biol. Pharm. Bull. 42: 989-995, 2019.
それ以前の代表的な発表論文
- Sasaki K, Tsuchiya T, and Osaki T: Isolation of Endogenous Peptides from Cultured Cell Conditioned Media for Mass Spectrometry. Methods Mol. Biol. 1719: 51-58, 2018.
- Tsuchiya T, Osaki T, Minamino N, and Sasaki K: Peptidomics for studying limited proteolysis. J. Proteome Res. 14: 4921-4931, 2015.
- Nonaka M, Kim R, Fukushima H, Sasaki K, Suzuki K, Okamura M, Ishii Y, Kawashima T, Kamijo S, Takemoto-Kimura S, Okuno H, Kida S, and Bito H: Region-Specific Activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron 84: 92-106, 2014.
- Sasaki K, Osaki T and Minamino N: Large-scale identification of endogenous secretory peptides using electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 12: 700-709, 2013.
- Nakamura M, Uehara Y, Asada M, Suzuki M and Imamura T: Sulfated glycosaminoglycan-assisted receptor specificity of human fibroblast growth factor (FGF) 19 signaling in a mouse system is different from that in a human system. J. Biomol. Screen. 18: 321-330, 2013.
- Shimamura S, Sasaki K and Tanaka M: The Src substrate SKAP2 regulates actin assembly by interacting with WAVE2 and cortactin proteins. J. Biol. Chem. 288: 1171-1183, 2013.
- Nagai N, Habuchi H, Sugaya N, Nakamura M, Imamura T, Watanabe H and Kimata K: Involvement of heparan sulfate 6-O-sulfation in the regulation of energy metabolism and the alteration of thyroid hormone levels in male mice. Glycobiology 23: 980-992, 2013.
- Osaki T, Sasaki K and Minamino N: Peptidomics-based discovery of an antimicrobial peptide derived from insulin-like growth factor-binding protein 5. J. Proteome Res. 10: 1870-1880, 2011.
- Nakamura M, Uehara Y, Asada M, Honda E, Nagai N, Kimata K, Suzuki M and Imamura T: Sulfated glycosaminoglycans are required for specific and sensitive fibroblast growth factor (FGF) 19 signaling via FGF receptor 4 and betaKlotho. J. Biol. Chem. 286: 26418-26423, 2011.
- Sasaki K, Takahashi N, Satoh M, Yamasaki M and Minamino N: A peptidomics strategy for discovering endogenous bioactive peptides. J. Proteome Res. 9: 5047-5052, 2010.
- Sasaki K, Satomi Y, Takao T and Minamino N: Snapshot peptidomics of the regulated secretory pathway. Mol. Cell. Proteomics 8: 1638-1647, 2009.
- Yamaguchi H, Sasaki K, Satomi Y, Shimbara T, Kageyama H, Mondal MS, Toshinai K, Date Y, González LJ, Shioda S, Takao T, Nakazato M and Minamino N: Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J. Biol. Chem. 282: 26354-26350, 2007.
- Sasaki K, Sato K, Akiyama Y, Yanagihara K, Oka M and Yamaguchi K: Peptidomics-based approach reveals the secretion of the 29-residue COOH-terminal fragment of the putative tumor suppressor protein DMBT1 from pancreatic adenocarcinoma cell lines. Cancer Res. 62: 4894-4898, 2002.